Flow cytometry troubleshooting involves identifying and resolving issues with data accuracy and instrument performance. Key areas include sample preparation, instrument settings, and data analysis.Flow cytometry is a powerful tool for analyzing cell populations. It helps researchers understand cellular properties and functions. However, ensuring accurate results can be challenging. Common problems include poor sample quality, incorrect instrument settings, and data interpretation errors. Addressing these issues promptly is crucial for reliable results.
Proper sample preparation, regular instrument calibration, and thorough data analysis are essential. By following best practices, researchers can optimize their flow cytometry experiments. This ensures precise and reproducible data, enhancing the overall quality of their research.
Introduction To Flow Cytometry
Flow cytometry is a powerful technique used in many research fields. It helps scientists study cells and particles in a fluid. This method is especially useful in immunology, cell biology, and cancer research.
What Is Flow Cytometry?
Flow cytometry is a technology that analyzes the properties of cells. It measures physical and chemical characteristics as cells flow in a fluid stream. The cells pass through a laser beam, and sensors detect their properties.
Here are the key components of a flow cytometer:
- Fluidics System: Transports the cells in a stream.
- Optics System: Includes lasers and detectors.
- Electronics System: Converts light signals into data.
Flow cytometry can analyze thousands of cells per second. This makes it a fast and efficient method.
Importance In Research
Flow cytometry plays a crucial role in many research areas. It allows researchers to perform detailed cell analysis. This can lead to new discoveries in disease mechanisms and treatments.
Here are some key applications:
- Immunophenotyping: Identifies different types of immune cells.
- Cell Sorting: Separates specific cell populations for further study.
- Apoptosis Detection: Measures cell death in response to treatments.
Flow cytometry provides high precision and accuracy. This makes it a valuable tool in modern research.
Sample Preparation Challenges
Flow cytometry requires precise sample preparation. Many face common issues during this stage. These challenges can affect your results significantly.
Cell Viability Issues
Cell viability is crucial in flow cytometry. Dead cells can skew results. Keep cells healthy throughout preparation.
Key steps to enhance cell viability:
- Use fresh samples whenever possible.
- Maintain cells at optimal temperature.
- Avoid harsh chemicals during washing steps.
- Use gentle pipetting techniques.
Check cell viability using Trypan Blue exclusion or similar assays. This will ensure more accurate flow cytometry data.
Debris And Clumping
Debris and clumping are major obstacles in flow cytometry. Debris can interfere with signal detection. Clumping can cause inaccurate cell counts.
Methods to reduce debris and clumping:
- Filter samples using a 40-70 µm mesh.
- Use DNase to minimize DNA-induced clumping.
- Perform gentle vortexing to break up clumps.
- Wash cells thoroughly to remove debris.
Consider using a table to track steps:
| Step | Action | Outcome |
|---|---|---|
| 1 | Filter samples | Removes large debris |
| 2 | Add DNase | Reduces clumping |
| 3 | Vortex gently | Breaks up clumps |
| 4 | Wash cells | Removes small debris |
Instrument Calibration
Proper instrument calibration is crucial for accurate flow cytometry results. Calibration ensures your instrument measures cell properties precisely. This section discusses common calibration issues and solutions.
Laser Alignment Problems
Laser alignment is vital for accurate detection. Misaligned lasers can cause data inaccuracies. Follow these steps to troubleshoot laser alignment problems:
- Check the laser beam’s path.
- Ensure the beam hits the center of the flow cell.
- Use alignment beads to verify the laser’s position.
A table can help summarize key points:
| Step | Description |
|---|---|
| 1 | Check laser beam path. |
| 2 | Ensure beam hits flow cell center. |
| 3 | Use alignment beads to verify position. |
Detector Sensitivity
Detector sensitivity affects signal detection and data quality. Ensure your detectors are functioning properly:
- Check the PMT voltage settings.
- Run quality control beads.
- Adjust the voltage for optimal signal.
High-quality detectors are essential for detecting weak signals. Regular calibration helps maintain detector performance. Use consistent quality control materials for best results.
Remember to document all calibration steps. This practice ensures reproducibility and accountability.
Data Acquisition Tips
Flow cytometry is a powerful tool for analyzing cells. Accurate data acquisition is crucial for reliable results. Below are some key tips to enhance your data acquisition process.
Optimizing Fluorescence
Optimizing fluorescence is essential for accurate data. Begin by selecting the right fluorochromes for your experiment.
- Use fluorochromes with minimal spectral overlap.
- Ensure the lasers in your cytometer match the fluorochromes.
- Adjust the voltage settings for each detector.
Proper calibration of your cytometer is also vital. Run calibration beads to standardize your instrument settings. This helps in achieving consistent results.
Compensation Techniques
Fluorescence compensation is necessary to correct spectral overlap. Follow these steps for effective compensation:
- Use single-stained controls for each fluorochrome.
- Adjust the compensation matrix based on these controls.
- Verify the compensation using multi-color controls.
Adjusting compensation settings correctly ensures accurate data. Below is a table that summarizes these steps:
| Step | Description |
|---|---|
| 1 | Use single-stained controls |
| 2 | Adjust the compensation matrix |
| 3 | Verify using multi-color controls |
Always double-check your compensation settings before acquiring data. This helps prevent errors and ensures data reliability.
Data Analysis Errors
Flow cytometry is powerful, but data analysis errors can skew results. Understanding these errors can improve your research accuracy.
Gating Strategy Mistakes
Gating is key in flow cytometry data analysis. Incorrect gating can lead to false results.
- Overlapping Populations: Ensure that cell populations do not overlap. This prevents misclassification.
- Incorrect Controls: Use proper controls to set gates correctly. This ensures data accuracy.
- Inconsistent Gates: Apply consistent gating across all samples. This maintains data integrity.
Statistical Misinterpretations
Statistical errors are common in flow cytometry analysis. Avoid these pitfalls for reliable data.
| Error | Description |
|---|---|
| Small Sample Size | Small samples can lead to inaccurate conclusions. |
| Incorrect Statistical Tests | Use the right tests for your data type. This ensures valid results. |
| Ignoring Variability | Always consider biological variability in your analysis. |
By understanding these errors, you can improve the accuracy of your flow cytometry data analysis.
Common Reagent Problems
Flow cytometry is a powerful tool for cell analysis. However, reagent problems can hinder your results. Common issues include improper antibody titration and poor fluorophore selection. Addressing these problems can enhance your data quality.
Antibody Titration
Proper antibody titration is crucial. Incorrect concentrations can lead to poor signal or high background. Always start with a small test panel. Use serial dilutions to find the optimal concentration.
Here is a simple table to guide you:
| Antibody Dilution | Expected Signal |
|---|---|
| 1:50 | High Signal |
| 1:100 | Optimal Signal |
| 1:200 | Low Signal |
Use the right buffer for antibody dilution. This minimizes non-specific binding. Always include a negative control. This helps to identify non-specific staining.
Fluorophore Selection
The right fluorophore selection is vital. Choose fluorophores with minimal overlap. This reduces compensation issues. Use single-stain controls to set compensation.
Consider the following tips:
- Use bright fluorophores for low-expressed markers.
- Match fluorophores to your flow cytometer’s lasers.
- Avoid using similar emission spectra fluorophores together.
Here’s a quick reference table:
| Fluorophore | Excitation Laser | Emission Spectrum |
|---|---|---|
| FITC | 488 nm | 519 nm |
| PE | 561 nm | 578 nm |
| APC | 633 nm | 660 nm |
Always verify the compatibility of fluorophores with your flow cytometer. This ensures accurate data collection.
Maintenance And Cleaning
Proper maintenance and cleaning of flow cytometry equipment ensure reliable results. Regular care extends the lifespan of the machine. Below are key steps for keeping your flow cytometer in top condition.
Preventive Maintenance
Preventive maintenance is essential for optimal performance. Follow these steps:
- Daily Checks: Inspect fluid levels and check for any leaks.
- Weekly Tasks: Verify laser alignment and clean the sample injection port.
- Monthly Maintenance: Perform a thorough inspection of all parts.
- Annual Servicing: Schedule professional servicing for detailed checks.
Preventive maintenance reduces the risk of unexpected failures. It also ensures consistent performance.
Decontamination Procedures
Decontamination is critical for accurate results. It prevents cross-contamination between samples. Follow these decontamination steps:
- Initial Rinse: Rinse the system with distilled water.
- Cleaning Solution: Use a recommended cleaning solution to flush the system.
- Final Rinse: Rinse again with distilled water to remove residues.
Regular decontamination keeps the flow cytometer clean. It ensures reliability and accuracy in your results.
| Maintenance Task | Frequency |
|---|---|
| Inspect Fluid Levels | Daily |
| Check for Leaks | Daily |
| Clean Sample Injection Port | Weekly |
| Verify Laser Alignment | Weekly |
| Full Inspection | Monthly |
| Professional Servicing | Annually |
Effective maintenance and cleaning practices are vital. They ensure your flow cytometer delivers accurate and reliable data every time.
Advanced Troubleshooting
Flow cytometry can be complex. Advanced troubleshooting ensures accurate results. This section covers two key areas: improving signal-to-noise ratio and resolving doublets.
Signal-to-noise Ratio
A high signal-to-noise ratio is crucial. It ensures clear data interpretation. Follow these tips to improve it:
- Use high-quality antibodies and reagents.
- Optimize your staining protocol.
- Ensure proper instrument calibration.
Adjust the voltage settings carefully. This can enhance signal strength. Always clean the instrument to avoid debris.
Use appropriate controls. They help distinguish between signal and noise. Include unstained and single-stained controls in your experiments.
Resolving Doublets
Doublets can distort your data. It’s essential to resolve them. Here are steps to follow:
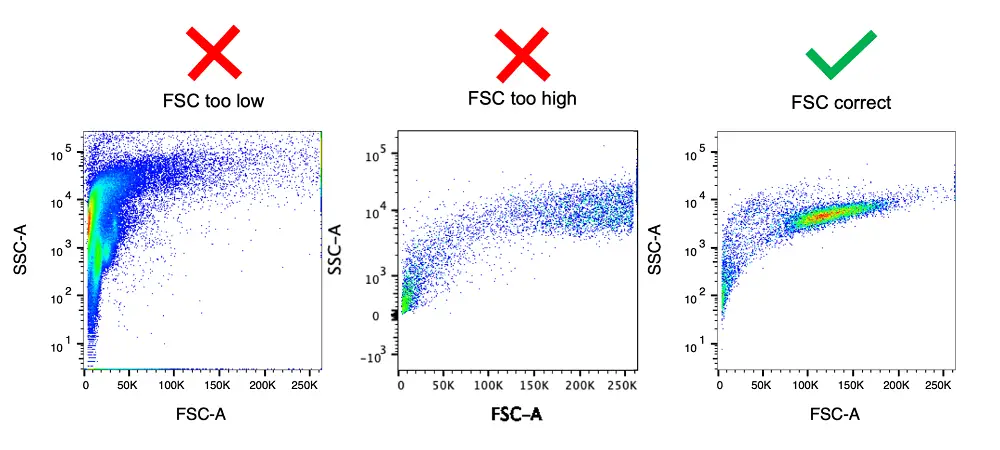
- Adjust the forward scatter (FSC) and side scatter (SSC) settings.
- Use doublet discrimination parameters (DDP).
- Gate on singlets using FSC-A vs. FSC-H or FSC-W.
Ensure the sample concentration is optimal. High concentration increases doublet formation. Always filter your samples to remove clumps.
| Step | Description |
|---|---|
| 1 | Adjust FSC and SSC settings |
| 2 | Use doublet discrimination parameters |
| 3 | Gate on singlets |
Regular maintenance of your flow cytometer is key. It prevents common issues and ensures reliable data.
Expert Tips And Best Practices
Flow cytometry is a powerful tool in research and clinical diagnostics.
To keep your data accurate and reliable, follow these expert tips and best practices.
This guide will cover routine checks, documentation, and record-keeping.
Routine Checks
Performing routine checks ensures your flow cytometer runs smoothly.
Regularly inspect all parts for wear and tear.
Clean the fluidics system weekly to prevent clogs and contamination.
Calibrate your machine daily to ensure consistent results.
Use standard beads to check the machine’s performance.
If issues arise, consult the machine’s manual for troubleshooting.
| Check | Frequency |
|---|---|
| Inspect Parts | Weekly |
| Clean Fluidics System | Weekly |
| Calibrate Machine | Daily |
Documentation And Record-keeping
Proper documentation and record-keeping are essential.
Keep a detailed log of your experiments.
Note the date, time, and conditions of each run.
Record any errors or issues you encounter.
Create a maintenance log for your machine.
Include dates of routine checks and any repairs done.
This helps track the machine’s performance over time.
- Log experiment details
- Note errors and issues
- Maintain a machine log
Following these best practices will help ensure your flow cytometry data is reliable.
Routine checks and proper documentation are key to successful experiments.
Frequently Asked Questions
How Do I Know If My Flow Cytometry Data Is Bad?
Check for high background noise, low signal-to-noise ratio, and poor resolution. Verify proper instrument calibration and control sample performance. Look for unexpected cell populations or anomalies in the data. Ensure data consistency across replicates.
Can Flow Cytometry Be Wrong?
Yes, flow cytometry can be wrong. Errors may occur due to sample preparation, instrument calibration, or data interpretation. Proper technique and regular maintenance reduce these risks.
How Do You Unclog A Flow Cytometer?
To unclog a flow cytometer, first flush the system with distilled water. Use a cleaning agent if necessary. Perform routine maintenance and inspect filters regularly to prevent future clogs.
How To Improve Flow Cytometry Results?
Optimize sample preparation and staining protocols. Use high-quality reagents and antibodies. Calibrate and maintain the flow cytometer regularly. Analyze data with appropriate software. Ensure proper gating strategies.
What Causes High Background In Flow Cytometry?
High background often results from non-specific binding or autofluorescence. Proper controls and antibody titration can help reduce it.
Conclusion
Mastering flow cytometry troubleshooting enhances your research accuracy and efficiency. Apply these tips to resolve common issues swiftly. Keep your protocols updated and equipment well-maintained. Regular practice and troubleshooting skills will ensure reliable results. Stay proactive and improve your flow cytometry experiments continuously.