Are you struggling with peak tailing in your HPLC results? You’re not alone.
Peak tailing can blur your chromatograms, making it hard to trust your data and quantify your samples accurately. But don’t worry—understanding why peaks tail and how to fix this issue can transform your analysis and boost your confidence in every run.
You’ll discover the common causes behind HPLC peak tailing and practical, easy-to-apply solutions. Whether the problem stems from chemical interactions or physical system issues, you’ll learn clear troubleshooting steps that will get your peaks sharp and symmetrical again. Keep reading to master peak tailing troubleshooting and take control of your chromatography results!
Causes Of Peak Tailing
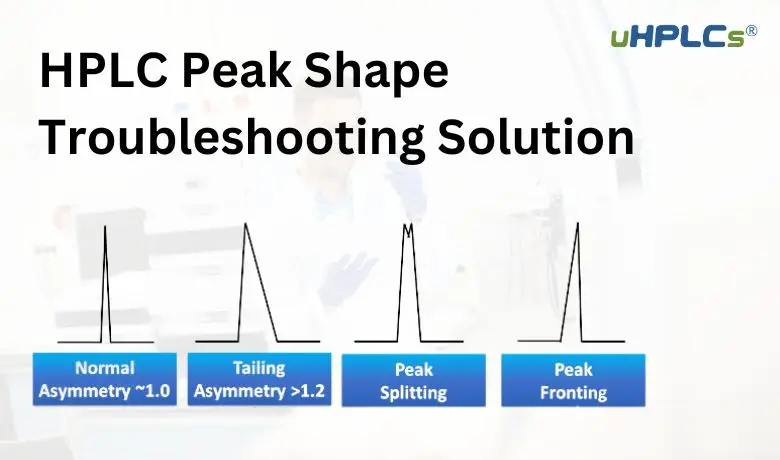
Peak tailing in HPLC reduces the clarity and accuracy of results. It happens when peaks are not symmetrical but stretch out to one side. Understanding the causes helps in fixing this issue quickly. Two main groups of causes explain why peak tailing occurs. These are chemical interactions and physical factors.
Chemical Interactions
Chemical interactions occur between the analyte and the stationary phase of the column. Acidic silanol groups on silica-based columns can react with basic analytes. This reaction slows some molecules more than others, causing tailing. Strong secondary interactions trap analyte molecules temporarily. Incomplete suppression of these active sites makes tailing worse.
Mobile phase pH also affects chemical interactions. If the pH is not optimized, analytes may bind too strongly to the column. Buffers and ion-pairing agents help reduce these effects. Sample solvent mismatch with the mobile phase can cause peak distortion. Diluting the sample or adjusting solvents may improve peak shape.
Physical Factors
Physical factors relate to the HPLC system and the column condition. Column voids or blockages create irregular flow paths. This causes uneven movement of molecules through the column. Dead volume from extra tubing or loose connections also leads to tailing peaks. Overloading the column with too much sample causes peak distortion.
Columns with damaged or degraded packing material increase tailing. Regular maintenance like backflushing or replacing the column is necessary. Ensuring the system is clean and free of obstructions improves peak symmetry. Proper system setup and sample loading prevent physical causes of tailing.
Credit: uhplcs.com
Chemical Issues And Fixes
HPLC peak tailing often stems from chemical problems within the system. These issues interfere with the smooth movement of analytes through the column. Fixing chemical causes is essential for sharp, symmetrical peaks. Below, common chemical issues and practical solutions are explained.
Acid-base Interactions
Strong acid-base interactions cause peak tailing in HPLC. Acidic silanol groups on the column can bind with basic parts of analytes. This binding slows some molecules more than others. The result is a tailing peak.
Adjusting the mobile phase pH helps reduce these interactions. Use buffers to keep pH stable and avoid extremes. Ion-pairing agents can block silanol groups and improve peak shape. Choosing columns with end-capped stationary phases also lowers acid-base problems.
Sample Solvent Mismatch
Using a sample solvent very different from the mobile phase causes tailing. Strong solvents can cause analytes to stick or spread unevenly. This leads to peak distortion.
Dilute samples in the mobile phase or a similar solvent. Avoid high-strength solvents in the injection. Matching solvent strength helps analytes move evenly through the column. This reduces tailing and improves peak symmetry.
Active Site Effects
Active sites on the column packing grab analytes and slow them down. These sites include metal impurities or exposed silanol groups. They cause uneven analyte interaction, creating tailing peaks.
Use columns with high-quality, well-treated packing. End-capping reduces exposed silanol groups and active sites. Conditioning the column with specific solvents can block active sites. Replace old or damaged columns to maintain good peak shapes.
Physical Problems And Solutions
Physical problems often cause peak tailing in HPLC analysis. These issues affect the flow and interaction of analytes within the system. Identifying and solving these physical problems improves peak shape and data quality. Below are common physical causes and their solutions.
Column Defects And Maintenance
Column defects like voids or cracks cause uneven flow paths. This leads to peak tailing and poor separation. Regular inspection and proper handling prevent damage. Backflushing the column can remove blockages inside. Replace the column if damage is severe. Store columns according to manufacturer instructions to maintain performance.
Mass Overload Effects
Injecting too much sample overloads the column. This saturates the stationary phase and causes tailing peaks. Use smaller injection volumes to avoid overload. Dilute samples if concentration is high. Follow the column’s capacity limits for best results. Overloading damages the column and reduces its lifespan.
System Plumbing Concerns
Leaks, dead volumes, and poorly connected tubing impact peak shape. Loose fittings cause pressure fluctuations and tailing. Use short, clean tubing with tight connections. Avoid extra dead volume by minimizing tubing length. Regularly check for leaks and replace damaged parts. Proper plumbing ensures smooth flow and sharp peaks.

Credit: www.youtube.com
Mobile Phase Optimization
Mobile phase optimization plays a key role in fixing HPLC peak tailing issues. The mobile phase interacts directly with the analytes and the column. Small changes in its composition can greatly improve peak shape. Careful selection and adjustment of buffers, ion-pairing agents, and organic modifiers can reduce tailing. This section discusses these factors and how to optimize them for better results.
Buffer Selection And Ph Control
Choosing the right buffer is critical to control peak tailing. Buffers stabilize the pH of the mobile phase. This reduces unwanted interactions between analytes and the column. Maintaining a pH that matches the analyte’s properties helps. It minimizes secondary bonding with the stationary phase. Using a buffer with good capacity ensures pH stays constant during the run. This improves peak symmetry and reduces tailing.
Ion-pairing Agents
Ion-pairing agents help neutralize charged analytes. They form complexes that move more uniformly through the column. This reduces interactions causing peak tailing. Selecting an ion-pair reagent that matches the analyte’s charge improves separation. Adjusting its concentration controls retention time and peak shape. Using too much ion-pairing agent can increase baseline noise. Finding the right balance is essential to reduce tailing and maintain signal clarity.
Organic Modifier Adjustments
Organic modifiers like methanol or acetonitrile affect peak tailing. They change the polarity and strength of the mobile phase. Increasing organic content often reduces analyte interaction with the stationary phase. This improves peak shape and shortens run time. However, too much organic solvent can cause peak fronting or loss of resolution. Gradual adjustment helps find the best ratio. Careful control of organic modifiers fine-tunes peak symmetry and reduces tailing.
Column Selection Tips
Choosing the right column is key to reducing peak tailing in HPLC. The column’s surface chemistry affects how analytes interact with the stationary phase. Selecting a column that minimizes unwanted interactions helps produce sharper, more symmetrical peaks. Below are some tips on column types that can improve peak shape and reduce tailing.
End-capped Columns
End-capped columns have silanol groups blocked by small groups. This reduces active sites that cause tailing with basic compounds. These columns help prevent strong secondary interactions. They are a good choice for samples with amines or other basic groups. Using end-capped columns often improves peak symmetry and reproducibility.
Base-deactivated Columns
Base-deactivated columns treat the silica surface to neutralize acidic sites. This reduces peak tailing caused by acidic silanols reacting with analytes. They work well with compounds sensitive to silanol activity. These columns offer stable retention and better peak shapes, especially for polar and ionic analytes.
Alternative Stationary Phases
Switching to different stationary phases can reduce tailing. Phases like phenyl, cyano, or polymer-based materials change interaction mechanisms. They often reduce unwanted secondary interactions. Try alternative phases when C18 columns cause persistent tailing. Different chemistry can improve peak shape and resolution for certain compounds.
Sample Preparation Strategies
Proper sample preparation plays a key role in reducing HPLC peak tailing. Poor preparation can cause strong interactions between the sample and the column. This leads to uneven flow and tailing peaks. Simple strategies can improve peak shape and accuracy. Focus on dilution, solvent choice, and filtration to get better results.
Dilution And Injection Volume
Diluting the sample lowers concentration and reduces overload on the column. High concentrations cause stronger interactions with the stationary phase. Smaller injection volumes also help prevent column saturation. This keeps the peaks sharp and symmetrical. Adjusting dilution and injection volume can fix many tailing problems quickly.
Solvent Compatibility
The sample solvent must match the mobile phase closely. A mismatch causes poor peak shapes and tailing. Strong solvents can disrupt the column’s chemistry. Use solvents that dissolve the sample but do not interfere with the stationary phase. Proper solvent compatibility ensures smooth analyte movement through the column.
Sample Filtration
Filtering the sample removes particles and impurities that block the column. Clogged columns cause uneven flow and peak tailing. Use filters with appropriate pore size to protect the column. Filtration also prevents damage and extends column life. Always filter samples before injection for reliable results.
Instrumental Adjustments
Instrumental adjustments play a vital role in fixing HPLC peak tailing. These adjustments help improve peak shape and overall chromatographic performance. Small changes in the instrument setup can reduce unwanted interactions and flow issues that cause peak tailing. Careful maintenance and tuning of the system parts keep the instrument running smoothly.
Below are key instrumental tips to troubleshoot and reduce peak tailing effectively.
Backflushing Columns
Backflushing helps clear blockages inside the column. It removes trapped particles and contaminants that cause peak tailing. This process pushes solvent in the reverse direction to clean the column bed. Backflushing extends column life and restores peak shape. Perform backflushing carefully and follow manufacturer guidelines to avoid damage.
Guard Columns And Filters
Guard columns protect the main column from dirt and impurities. They trap particles before they reach the analytical column. Using a guard column reduces column clogging and peak tailing. Regularly replace guard columns and inline filters. Clean filters prevent debris from entering the system and causing flow issues. Well-maintained filters improve peak symmetry and system reliability.
Tubing And Fittings Check
Inspect tubing and fittings for leaks or blockages. Loose or damaged connections cause pressure changes and flow disturbances. These issues lead to poor peak shapes and tailing. Replace worn tubing and tighten fittings securely. Use proper tubing sizes to reduce dead volume. A well-maintained flow path ensures consistent pressure and smooth peak elution.
Monitoring And Preventive Practices
Monitoring and preventive practices play a key role in reducing peak tailing in HPLC. Consistent care and checks help maintain column performance and system reliability. Small actions done regularly prevent bigger problems later. These practices ensure sharper peaks and more accurate results.
Routine Column Care
Proper column care extends the life of your HPLC column. Flush the column with suitable solvents after each run. Avoid strong solvents that can damage the stationary phase. Store the column in a recommended solvent to prevent drying. Use guard columns to protect the main column from contamination. Replace the guard column regularly to maintain effectiveness.
System Pressure Monitoring
Monitor system pressure daily to detect blockages or leaks early. Sudden pressure changes can signal column clogging or tubing issues. Keep pressure within the recommended range to avoid damaging the column. Record pressure trends to spot gradual changes over time. Address pressure problems immediately to avoid poor peak shapes.
Regular Method Evaluation
Review your HPLC method periodically to ensure optimal performance. Check mobile phase composition and pH for consistency. Verify sample preparation steps to avoid introducing contaminants. Adjust flow rate and temperature if peak tailing reappears. Update method parameters based on system performance and sample changes.

Credit: uhplcs.com
Frequently Asked Questions
What Causes Peak Tailing In Hplc?
Peak tailing in HPLC occurs due to strong interactions between analytes and residual silanol groups on the column. These interactions cause uneven analyte travel speeds. Other causes include sample solvent mismatch, column voids, or overloading, leading to asymmetric peak shapes and reduced quantification accuracy.
How To Fix Tailing On Hplc?
Fix HPLC tailing by using end-capped columns, optimizing mobile phase pH, adding buffers or ion-pairing agents. Match sample solvent strength to mobile phase and reduce injection volume. Check for column voids, blockages, or tubing issues; clean or replace the column if needed.
Why Is Peak Tailing A Problem?
Peak tailing distorts chromatogram quality, reduces quantification accuracy, and lowers confidence in analytical results. It hinders precise component identification.
What Is An Acceptable Tailing Factor?
An acceptable tailing factor ranges from 1. 0 to 2. 0. Values above 2. 0 indicate poor peak symmetry and require troubleshooting.
What Causes Peak Tailing In Hplc Analysis?
Peak tailing happens due to strong interactions between analytes and the column’s active sites. Acidic silanol groups on the column can bind with basic parts of the analyte, slowing some molecules. This uneven travel causes the chromatographic peaks to tail.
Conclusion
Peak tailing in HPLC can affect your results and reduce accuracy. Fixing it takes patience and careful checks. Adjust the mobile phase pH and use proper buffers. Choose the right column and avoid sample overload. Clean or replace parts to remove blockages and voids.
Small changes can improve peak shapes significantly. Keep testing and refining your method step by step. This helps ensure reliable and clear chromatograms every time.