Polymerase Chain Reaction (PCR) is a crucial technique in molecular biology. It amplifies DNA, making it essential for research, diagnostics, and more.
But what happens when PCR doesn’t work as expected? Troubleshooting PCR can be challenging, even for experienced scientists. Errors can arise from various sources, such as contamination, incorrect temperatures, or poor primer design. Understanding these potential issues is key to successful PCR experiments.
In this post, we will explore common PCR problems and provide practical solutions. This guide aims to help you identify and resolve issues, ensuring reliable and reproducible results. Whether you’re a novice or a seasoned researcher, these tips will enhance your PCR troubleshooting skills. Let’s dive in and make your PCR runs more successful!
Common Pcr Problems
Polymerase Chain Reaction (PCR) is a powerful tool in molecular biology. Yet, it can be frustrating when things go wrong. Here, we will explore some common PCR problems and how to troubleshoot them.
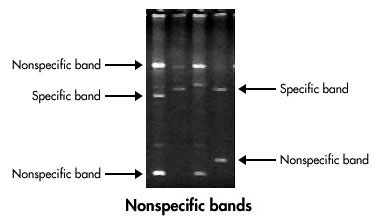
Non-specific Bands
Non-specific bands appear when PCR amplifies unintended DNA sequences. They can make it hard to interpret results. Here are some possible causes and solutions:
- Primer Design: Poorly designed primers can bind to unintended sites. Ensure primers are specific to your target sequence.
- Annealing Temperature: Too low an annealing temperature can cause non-specific binding. Increase the temperature in small steps.
- Magnesium Concentration: High magnesium levels can increase non-specific binding. Adjust the magnesium concentration accordingly.
- Cycling Conditions: Too many cycles can amplify non-specific products. Reduce the number of cycles if necessary.
No Bands
Sometimes, you might not see any bands on your gel. This can be frustrating but is often fixable. Consider these potential issues:
| Problem | Solution |
|---|---|
| Template DNA Quality: | Check the quality and quantity of your DNA. Degraded DNA can lead to no amplification. |
| Primer Design: | Ensure your primers are correctly designed and synthesized. They should match your target sequence. |
| PCR Components: | Verify that all PCR components are added correctly. Missing or degraded reagents can cause no amplification. |
| Cycling Conditions: | Check the cycling conditions. Incorrect temperatures or times can result in no bands. |
Troubleshooting PCR can be challenging, but understanding common problems helps. Pay attention to primer design, reagent quality, and cycling conditions. These steps ensure successful PCR results.
Optimizing Pcr Conditions
Polymerase Chain Reaction (PCR) is a powerful tool in molecular biology. But sometimes, the results are not as expected. Optimizing PCR conditions can help improve the outcome. This section explores two crucial factors: annealing temperature and magnesium concentration.
Annealing Temperature
The annealing temperature is crucial for the success of PCR. If the temperature is too low, non-specific binding can occur. If too high, primers may not bind efficiently. Finding the optimal temperature is a balancing act. Use a gradient PCR to find the best temperature for your primers.
Here is a simple guide to help:
- Start with the melting temperature (Tm) of your primers.
- Subtract 2-5°C from the Tm.
- Run a gradient PCR around this temperature.
Magnesium Concentration
Magnesium concentration is another key factor. Magnesium ions are co-factors for the Taq polymerase enzyme. Low magnesium can reduce enzyme activity. High magnesium can increase non-specific amplification. Balancing the magnesium concentration is essential.
Follow these steps to optimize:
- Start with a standard concentration of 1.5 mM.
- Prepare a series of reactions with different magnesium concentrations.
- Test concentrations ranging from 1.0 mM to 4.0 mM.
Compare the results to find the optimal concentration for your PCR.
Template Quality
Template quality significantly impacts PCR success. High-quality templates ensure efficient amplification. Poor quality can lead to inconsistent results. Understanding template quality involves examining DNA purity and template quantity.
Dna Purity
DNA purity is crucial for PCR. Contaminants like proteins and chemicals can inhibit the process. Pure DNA leads to better amplification. Use spectrophotometry to check DNA purity. Ideal ratios are 1.8-2.0 for A260/A280. Ratios below 1.8 suggest protein contamination. Ratios above 2.0 indicate possible RNA contamination.
Template Quantity
Template quantity affects PCR efficiency. Too much DNA can cause non-specific products. Too little DNA may lead to weak signals. Use the right amount for optimal results. Typically, 1-10 ng of template per reaction is recommended. Adjust based on the template and PCR conditions. Accurate quantification is essential for consistency.

Credit: www.biocompare.com
Primer Design Tips
Effective primer design is crucial for successful PCR experiments. Primers are short sequences of nucleotides that provide a starting point for DNA synthesis. Incorrectly designed primers can lead to PCR failure. Here are some essential tips for designing primers.
Primer Length
The length of primers plays a significant role in PCR efficiency. Ideally, primers should be between 18-24 nucleotides long. This length ensures specificity while maintaining stability. Primers that are too short may bind non-specifically, causing unwanted amplification. On the other hand, longer primers can form secondary structures, which hinder the PCR process.
Gc Content
GC content refers to the percentage of guanine (G) and cytosine (C) bases in the primer. Optimal GC content ranges from 40% to 60%. This balance ensures proper binding strength and stability. Higher GC content increases melting temperature, which can affect primer binding. Lower GC content may lead to weak binding and inefficient amplification.
| Aspect | Recommendation |
|---|---|
| Primer Length | 18-24 nucleotides |
| GC Content | 40%-60% |
- Avoid sequences with high homology to other regions.
- Prevent runs of identical nucleotides.
- Ensure primers do not form hairpins or dimers.
Following these tips can enhance PCR success, leading to accurate and reliable results.
Enzyme Selection
Choosing the right enzyme is crucial for successful PCR troubleshooting. Different enzymes have unique properties that can influence your results. This section covers the importance of selecting the appropriate enzyme for your specific needs.
Hot-start Taq
Hot-start Taq enzymes are designed to reduce non-specific amplification. These enzymes are inactive at room temperature and require a heat activation step. This prevents unwanted reactions during the setup phase.
The activation step usually occurs at 95°C for 2-10 minutes. This ensures that the enzyme is fully active only when the reaction temperature is high. This specificity helps in obtaining clearer and more accurate results.
High-fidelity Enzymes
High-fidelity enzymes are essential for applications where accuracy is critical. These enzymes have a proofreading ability that corrects errors during DNA synthesis. This results in fewer mutations and higher accuracy.
High-fidelity enzymes are especially useful for cloning and sequencing applications. They ensure that the amplified DNA is as close to the original sequence as possible. These enzymes are generally more expensive, but their benefits often outweigh the cost.
Here’s a quick comparison table for better understanding:
| Enzyme Type | Key Feature | Application |
|---|---|---|
| Hot-start Taq | Reduces non-specific amplification | General PCR, diagnostics |
| High-fidelity | High accuracy, proofreading ability | Cloning, sequencing |
Understanding the specific properties of these enzymes can significantly improve your PCR troubleshooting efforts. Choose wisely based on your experiment’s requirements.
Credit: www.bio-rad.com
Contamination Prevention
Contamination in PCR can lead to false results. Preventing contamination is crucial for accurate outcomes. A few simple steps can help maintain a clean environment and ensure reliable data.
Clean Work Area
Always start with a clean workspace. Clean surfaces with disinfectant before starting. Use dedicated pipettes and tips for PCR work only. Avoid sharing these tools with other tasks.
Wear gloves and change them frequently. This minimizes the risk of contamination from your hands. Use sterile tubes and reagents. Keep them closed when not in use.
Use Of Controls
Include controls in each PCR run. Positive controls confirm the PCR is working. Negative controls check for contamination. Use no-template controls (NTC) to ensure no contamination in reagents.
Analyze controls first. This helps identify any issues early. If controls fail, troubleshoot before analyzing samples. This saves time and ensures accuracy.
Pcr Additives
PCR (Polymerase Chain Reaction) can sometimes be tricky. Various additives can enhance its performance. These additives can help with amplification efficiency, specificity, and yield. Below, we discuss some common PCR additives.
Dmso
DMSO (Dimethyl Sulfoxide) is a common PCR additive. It helps with the denaturation of DNA. DMSO is especially useful for GC-rich templates. GC-rich regions can form strong secondary structures. These structures can hinder the progress of the polymerase. Adding DMSO can help to break these structures. This makes the DNA more accessible for amplification.
Using DMSO can improve the yield of PCR products. It can also increase the specificity of the reaction. Typically, a concentration of 5-10% DMSO is used. But, the optimal concentration may vary. It is important to test different concentrations. This will help to find the best conditions for your PCR reaction.
Bsa
BSA (Bovine Serum Albumin) is another helpful PCR additive. It stabilizes the enzyme during the PCR process. BSA can bind to inhibitors present in the reaction. These inhibitors can come from the DNA template or other sources. By binding to inhibitors, BSA prevents them from affecting the PCR reaction.
Adding BSA can increase the efficiency of the PCR. It can also improve the yield of the amplified product. A typical concentration is 0.1-1 µg/µl. As with DMSO, it is important to test different concentrations. This helps to determine the best conditions for your specific PCR setup.
Credit: www.bio-rad.com
Troubleshooting Guide
Polymerase Chain Reaction (PCR) is a powerful tool in molecular biology. But even experienced scientists encounter issues. This troubleshooting guide helps solve common PCR problems. Follow these steps to improve your PCR results.
Flowchart
A flowchart simplifies PCR troubleshooting. Begin by checking the reagents. Ensure you use fresh dNTPs, primers, and enzymes. Next, verify the thermal cycler settings. Confirm the correct annealing temperature and cycle number. Finally, check the template DNA quality. High-quality DNA ensures better results.
Common Issues And Fixes
PCR problems often fall into a few categories. Here, we address the most common issues and their solutions.
No Amplification: Ensure all reagents are added. Check primer design. Optimize the annealing temperature. Increase the template DNA amount.
Non-Specific Bands: Lower the annealing temperature. Use hot-start Taq polymerase. Design better primers. Increase the annealing time.
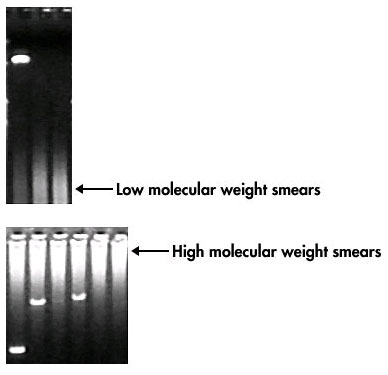
Smearing: Reduce the cycle number. Optimize magnesium concentration. Ensure the DNA template is pure. Check the gel electrophoresis conditions.
Primer-Dimer Formation: Lower primer concentration. Increase the annealing temperature. Use a touchdown PCR method.
Weak Bands: Increase the number of cycles. Increase the template DNA amount. Optimize the primer concentration.
By following this guide, you can solve most PCR issues. Ensure each step is carefully checked. Happy troubleshooting!
Frequently Asked Questions
What Causes Pcr To Fail?
PCR can fail due to poor primer design, contamination, or incorrect temperatures.
How Can I Avoid Pcr Contamination?
Use clean pipettes, wear gloves, and work in a clean area to avoid contamination.
Why Is My Pcr Yield Low?
Low PCR yield may result from insufficient template DNA or suboptimal reaction conditions.
What Can I Do If I Get Non-specific Bands?
Optimize annealing temperature or redesign primers to reduce non-specific bands.
How Important Is The Quality Of Template Dna?
High-quality DNA is crucial for successful PCR. Impurities can inhibit the reaction.
Conclusion
Effective PCR troubleshooting ensures successful experiments. Simple steps can resolve common issues. Check each component carefully. Maintain proper temperatures and timings. Always use high-quality reagents. Regularly calibrate equipment for accuracy. Document every step meticulously. Consistency in practice leads to reliable results.
Testing small changes can help identify problems. Stay patient and persistent. Troubleshooting is a skill that improves with experience. Happy experimenting!






