If you’re experiencing issues with qPCR, such as no amplification or high or low Ct values, potential causes may include limiting reagents, degraded reagents, inefficient reactions, or incorrect probe concentrations. Troubleshooting PCR and RT-PCR amplification can involve checking sample quality, primer design, and concentration, and using dedicated software for primer design.
It’s important to ensure that primers are present in excess and at equal concentrations in the reaction mixture. Additionally, contamination, baseline adjustment starting too early, and poor efficiency during PCR can also contribute to troubleshooting challenges. Matching qPCR amplification curves to commonly seen suboptimal data can help identify issues and find solutions.
Seek assistance from resources like the Real-Time PCR Doctor or refer to qPCR troubleshooting guides and videos for further support.
No Amplification
Potential Causes for No Amplification:
1. Limiting reagents
The first potential cause of no amplification in qPCR troubleshooting could be limiting reagents. This means that one or more of the necessary components in the reaction mixture may not be available in sufficient quantities to support amplification. Limiting reagents can include primers, dNTPs, or master mix components.
2. Degraded reagents (dNTPs or master mix)
Another potential cause of no amplification is degraded reagents, such as dNTPs or master mix. Over time, these reagents can degrade, leading to reduced or no amplification. It is important to check the quality and expiration date of these reagents before starting the qPCR reaction.
3. Some probe dyes are less bright than others
It is also possible that the probe dye used in the reaction may be less bright than others. This can result in lower signal intensity, making it difficult to detect amplification. If this is the case, it may be necessary to switch to a different probe dye with a higher brightness for better results.
4. Inefficient reaction
Inefficiency in the reaction can also lead to no amplification. This can be caused by a variety of factors, such as suboptimal primer design or concentration, low-quality primers, or incompatible reaction conditions. It is important to optimize these parameters to ensure successful qPCR amplification.
High Ct Values
High Ct values in qPCR can indicate potential issues with the amplification of the target sequence. Ct values, or cycle threshold values, represent the number of cycles needed for the fluorescent signal to reach a detectable level. The higher the Ct value, the lower the amount of target sequence present in the sample.
Potential Causes:
Faulty Primer Design
Faulty primer design can lead to high Ct values in qPCR. It is crucial to ensure that the primers are designed properly and are specific to the target sequence. Mismatches or nonspecific binding can result in inefficient amplification and higher Ct values.
Suboptimal Primer Concentration
The concentration of primers in the PCR reaction can impact the efficiency of amplification. If the primer concentration is too low, it can result in less efficient amplification and higher Ct values. Conversely, if the primer concentration is too high, it can lead to nonspecific amplification and inaccurate results.
Low-Quality Primers
The quality of the primers used in qPCR is paramount to achieving accurate results. Low-quality primers can have various issues, such as degradation, nonspecific binding, or incorrect sequences. These issues can all contribute to higher Ct values in the qPCR assay.
To address these potential causes of high Ct values, several steps can be taken:
- Use dedicated software for primer design to ensure accurate and specific primer sequences.
- Check the primer quality on a denaturing polyacrylamide gel to ensure they are intact and free from degradation.
- Check the PCR product on an agarose gel to verify the specificity of the amplification, ensuring there are no non-specific bands.
- Ensure that the primers are present in excess in the reaction mixture and are at equal concentrations. This helps to ensure efficient amplification and consistent results.
By addressing these potential causes of high Ct values and taking appropriate troubleshooting measures, one can improve the quality and accuracy of qPCR results.
Low Ct Values
Potential Causes:
When performing qPCR, obtaining low Ct values is often desirable as it indicates a higher abundance of the target sequence in the sample. However, sometimes low Ct values can raise concerns and require troubleshooting to ensure accurate and reliable results. Here are some potential causes for low Ct values:
Contamination From Laboratory Exposure To The Same Target Sequence
Contamination can be a common issue in the laboratory, particularly when working with highly sensitive techniques like qPCR. If laboratory surfaces, reagents, or equipment have been exposed to the target sequence, it can lead to false-positive results and low Ct values. It is essential to follow strict laboratory practices, including dedicated work areas, separate materials, and thorough cleaning procedures, to prevent contamination.
Baseline Adjustment Is Starting Too Early
Baseline adjustment is a critical step in qPCR analysis, as it determines the threshold for Ct value calculation. If the baseline adjustment is set too early, it can result in artificially low Ct values. Ensure that the baseline adjustment is correctly positioned to capture the exponential amplification phase of the PCR curve, providing accurate Ct values.
Poor Efficiency During Pcr
Poor PCR efficiency can lead to low Ct values, as it indicates inefficient amplification of the target sequence in the sample. Several factors can contribute to poor PCR efficiency, including suboptimal primer design, degraded reagents, inadequate reaction conditions, or incorrect probe concentration. Optimizing these factors and verifying primer quality can improve PCR efficiency and yield more reliable Ct values.
In conclusion, low Ct values in qPCR can arise due to contamination, baseline adjustment errors, or poor efficiency during PCR. By addressing these potential causes, researchers can troubleshoot low Ct values and ensure the accuracy and reliability of their qPCR results.
Troubleshooting Tips
When it comes to qPCR troubleshooting, finding the root cause of the issue can be challenging. However, by following some key troubleshooting tips, you can effectively identify and resolve the problems in your qPCR experiments. Here are some important steps to consider:
Check The Quality Of The Sample (degraded Material Will Cause Erroneous Results)
In qPCR, the quality of the sample is crucial for obtaining accurate results. Any degraded material present in the sample can lead to erroneous amplification curves. To ensure the quality of your sample, follow these steps:
- Verify the integrity of the RNA or DNA before performing qPCR.
- Use high-quality RNA or DNA extraction methods to minimize degradation.
- Check the concentration and purity of the extracted nucleic acids using a spectrophotometer.
Check If The Rt Protocol Is Compatible With The Design
The reverse transcription (RT) step is an essential part of qPCR for converting RNA into complementary DNA (cDNA). However, using an incompatible RT protocol can lead to inaccuracies in your qPCR results. To troubleshoot this issue, consider the following:
- Ensure that the RT enzyme and buffers used are compatible with the primer and probe design.
- Optimize the RT reaction conditions, such as temperature and incubation time, to achieve efficient cDNA synthesis.
- Validate the RT reaction by including positive and negative controls.
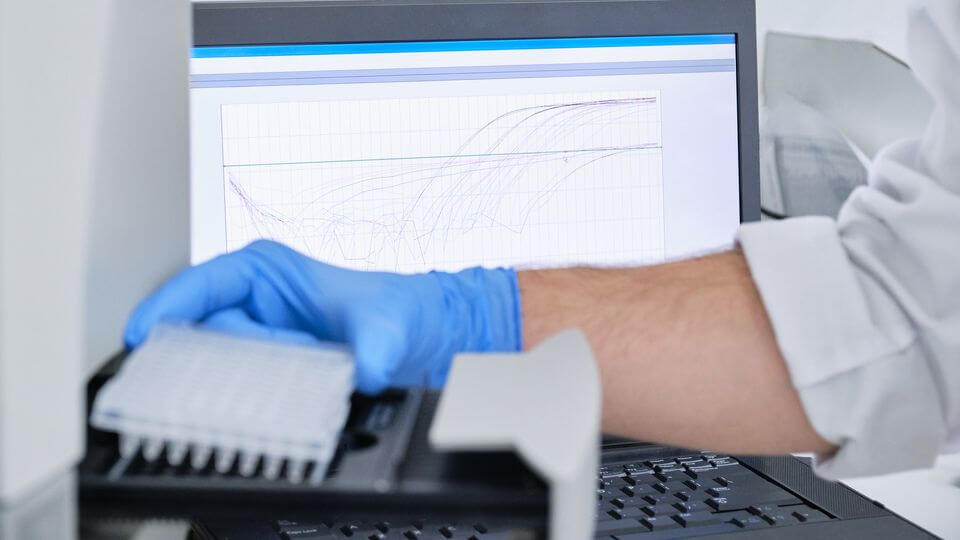
Interpretation Of Amplification Curves
The amplification curves obtained during qPCR experiments provide valuable information about the efficiency and specificity of the reaction. However, improper interpretation of these curves can lead to misdiagnosis of the results. Here’s how you can troubleshoot any unexpected amplification curves:
- Check the baseline adjustment and ensure it is starting at the appropriate cycle.
- Evaluate the shape of the amplification curves. A well-defined sigmoidal shape indicates successful amplification, while irregular or skewed curves may indicate issues.
- Assess the amplification efficiency by analyzing the slope of the curve. Low efficiency can be indicative of suboptimal reaction conditions.
By following these troubleshooting tips, you can quickly identify and resolve common issues in qPCR experiments. Remember to validate and optimize each step of your protocol to ensure reliable and accurate results.
Resources For Qpcr Troubleshooting
When it comes to qPCR troubleshooting, having access to the right resources can save you time and frustration. Whether you’re facing issues with amplification curves or contamination prevention, there are several resources available to help you identify and resolve these problems effectively. In this section, we will be highlighting a few key resources that can provide valuable guidance in troubleshooting qPCR experiments.
Technical Support From Biotechrabbit
If you need technical support for qPCR troubleshooting, Biotechrabbit offers a comprehensive service to assist you. Their team of experts can guide primer design, optimization techniques, and troubleshooting strategies. With their assistance, you can quickly identify and address any issues that may arise during your qPCR experiments.
Pcr Troubleshooting Checklist From Sigma-Aldrich
Sigma-Aldrich offers a PCR troubleshooting checklist that can serve as a helpful reference when encountering problems in your qPCR experiments. This checklist covers a variety of potential issues, including inadequate sample quality, primer design problems, and reaction optimization. By following this checklist, you can systematically troubleshoot your experiment and identify the root causes of any difficulties you may be facing.
Troubleshooting Examples From Sigma-Aldrich
In addition to their PCR troubleshooting checklist, Sigma-Aldrich also provides troubleshooting examples that showcase common issues encountered in qPCR experiments. These examples offer step-by-step guidance on how to diagnose and resolve problems such as poor amplification efficiency and baseline adjustments. By referring to these troubleshooting examples, you can gain valuable insights into the best approaches for resolving specific issues in your qPCR experiments.
Blog Post On Qpcr Troubleshooting: Interpreting Amplification Curves And Contamination Prevention By Lgc Biosearch Technologies
LGC Biosearch Technologies has published a helpful blog post that focuses on interpreting amplification curves and contamination prevention in qPCR experiments. This blog post delves into the intricacies of analyzing amplification curves and provides insights into preventing and managing contamination issues. By reading this blog post, you can gain a better understanding of how to interpret your qPCR results and implement effective contamination prevention strategies.
In conclusion, these resources provide valuable guidance and support for qPCR troubleshooting. Whether you need technical assistance, a comprehensive checklist, troubleshooting examples, or in-depth insights on specific issues, these resources can help you overcome challenges and optimize your qPCR experiments.
Frequently Asked Questions
What Can Go Wrong In Qpcr?
Potential issues in qPCR include limiting reagents, degraded reagents, suboptimal primer design or concentration, low-quality primers, and inefficient reactions. It’s important to use dedicated software for primer design, check primer and PCR product quality, and ensure excess primer concentrations in the reaction mixture.
Contamination and poor baseline adjustment can also affect qPCR results. Troubleshooting guides and expert support are available for assistance.
What Are The Common Troubleshooting That Can Occur In Pcr?
Common PCR troubleshooting issues include limited reagents, degraded reagents, inefficient reactions, incorrect probe concentrations, faulty primer design, suboptimal primer concentrations, and contamination. These issues can lead to no amplification, high or low Ct values, and unexpected amplification curves.
Why isn’t my QPCR working?
There could be several reasons why your qPCR is not working. It could be due to faulty primer design, suboptimal primer concentration, or low-quality primers. Ensure you use dedicated software for primer design and check primer quality on denaturing polyacrylamide gel.
It’s important to have excess primers in the reaction mixture and at equal concentrations.
Why Is My Qpcr Not Amplifying?
There are several potential causes for qPCR not amplifying: limiting reagents, degraded reagents, inefficient reaction, incorrect probe concentration, faulty primer design, suboptimal primer concentration, and low-quality primers. It is important to use dedicated software for primer design, check primer quality on denaturing polyacrylamide gel and the PCR product on agarose gel, and ensure that primers are present in excess and at equal concentrations in the reaction mixture.
How Can I Troubleshoot My Qpcr When There Is No Amplification?
In some cases, limiting reagents or degraded reagents could be the cause of no amplification. Additionally, it’s important to check the probe concentration and probe dye brightness.
Conclusion
Troubleshooting qPCR can be a daunting task, but with the right techniques and knowledge, it is possible to overcome common issues. By checking for limiting or degraded reagents, ensuring optimal primer design and concentration, and utilizing specialized software, you can improve the efficiency and accuracy of your qPCR experiments.
Additionally, understanding potential causes such as contamination and baseline adjustment can help you troubleshoot unexpected amplification curves. Remember to consult reliable resources and seek professional help when needed. With practice and experience, you can master qPCR troubleshooting and achieve successful results.